Bergamaschi L, et al. (2021) Delayed bystander CD8 T cell activation, early immune pathology and persistent dysregulation characterise severe COVID-19. medRxive. https://doi.org/10.1101/2021.01.11.20248765

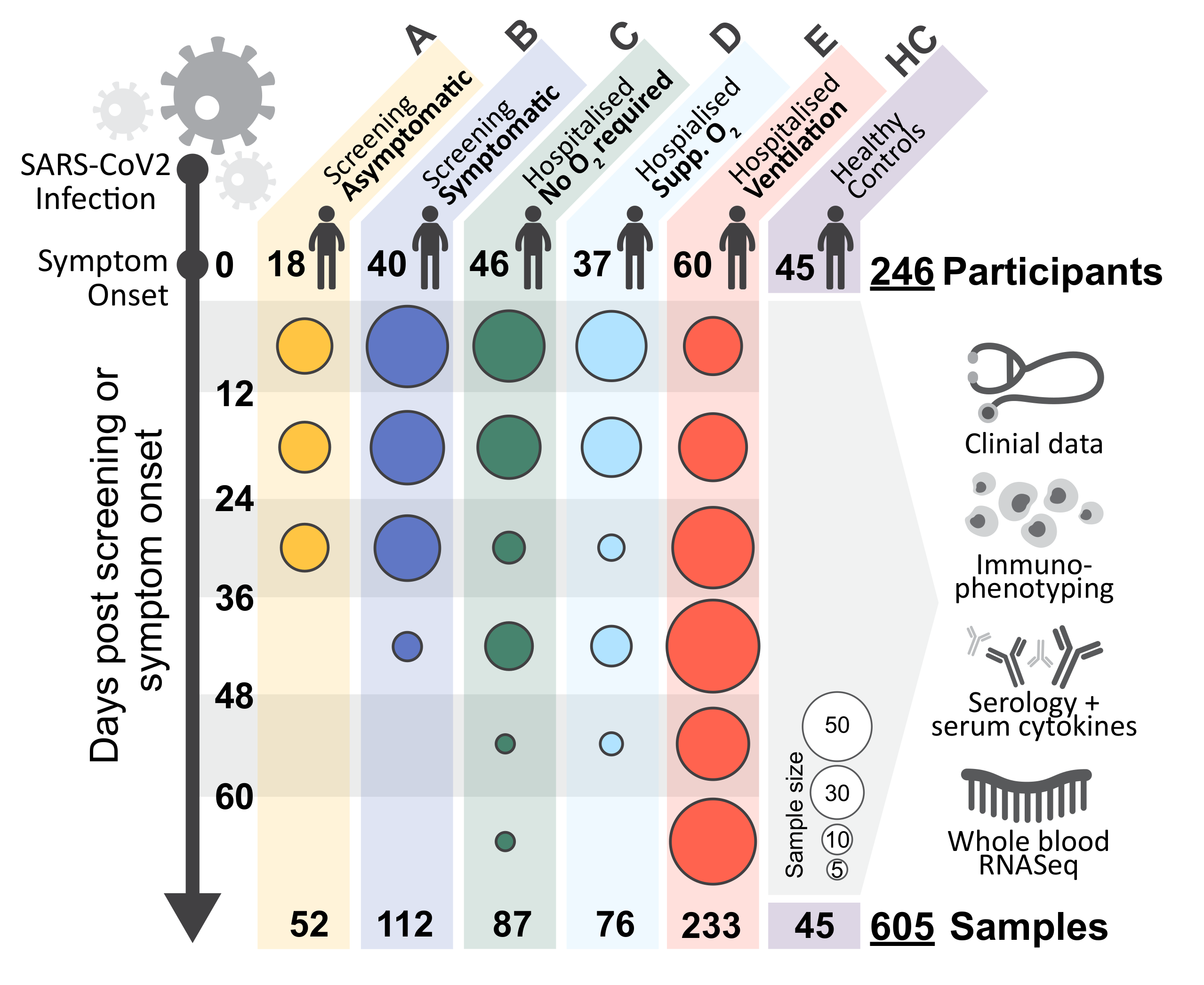
In a study of 207 SARS-CoV2-infected individuals with a range of severities followed over 12 weeks from symptom onset, we demonstrate that an early robust bystander CD8 T cell immune response, without systemic inflammation, is characteristic of asymptomatic or mild disease. Those presenting to hospital had delayed bystander responses and systemic inflammation already evident at around symptom onset. Such early evidence of inflammation suggests immunopathology may be inevitable in some individuals, or that preventative intervention might be needed before symptom onset. Viral load does not correlate with the development of this pathological response, but does with its subsequent severity. Immune recovery is complex, with profound persistent cellular abnormalities correlating with a change in the nature of the inflammatory response, where signatures characteristic of increased oxidative phosphorylation and reactive-oxygen species-associated inflammation replace those driven by TNF and IL-6. These late immunometabolic inflammatory changes and unresolved immune defects may have clinical implications.
One entry per participant
| Patient_ID | Study participant ID |
|---|---|
| Disease_status | COVID19 case or swab-negative control |
| Age | Age at study recruitment |
| Gender | Self reported gender |
| COVID19_swab_PCR | COVID19 swab PCR outcome at study recruitment (controls were confirmed COVID19 serology negative, see Methods) |
| CT_first_pos_swab | COVID19 swab PCR cycle threshold |
| COVID19_symptoms | Symptomatic or asymptomatic infection (with or without previous symptoms) at study recruitment. Note, previous symptoms in asymptomatic participants could not be confirmed COVID19-specific. |
| Max_resp_support | Maximum respiratory support over course of follow-up |
| Hospital_outcome | Deceased or alive at end of hospital admission or study follow up period |
| Severity_group | Disease severity classification based on maximum respiratory support |
| Severity_group_paper | Alphabetised severity classification used in publication |
Multiple entries per participant
| Patient_ID | Study participant ID |
|---|---|
| Sample_ID | Sample ID, incorporating approximate day of follow-up from study design |
| Days_from_symptom_or_swab | Days between reference date (group A, date of first positive swab; groups B-E, date of self-reported symptom onset) and follow up |
| Current_resp_support | Current respiratory support on day of follow up |
| hsCRP_mg/L | High-sensitivity C-reactive protein measure (mg/L) |
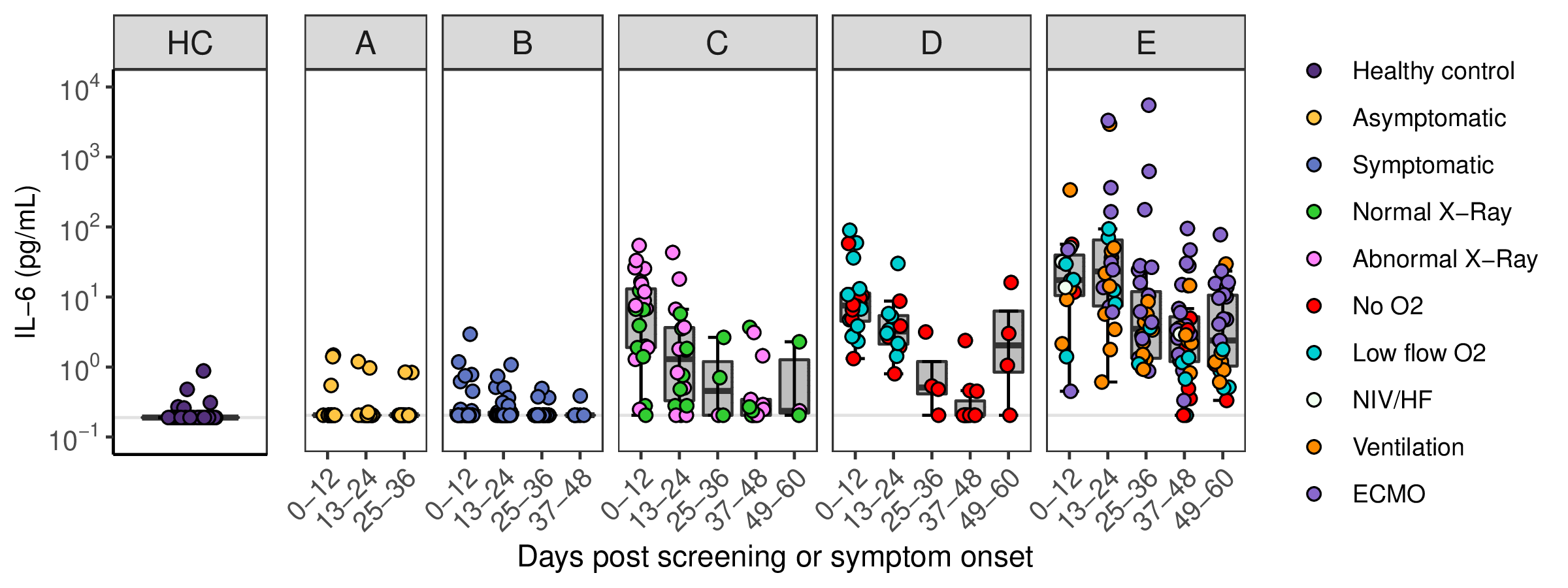
| Sample_ID | Sample ID, incorporating approximate day of follow-up from study design (see FILE 1 and 2 for metadata) |
|---|---|
| IL6_pg.mL | Interleukin 6 concentration (pg/mL) measured by standard clinical assay (Addenbrooke's Hosptial) |
| TNFa_pg.mL | Tumour necrosis factor aplpha concentration (pg/mL) measured by standard clinical assay (Addenbrooke's Hosptial) |
| IL1B_pg.mL | Interleukin 1 beta concentration (pg/mL) measured by standard clinical assay (Addenbrooke's Hosptial) |
| IL10_pg.mL | Interleukin 10 concentration (pg/mL) measured by standard clinical assay (Addenbrooke's Hosptial) |
| IFNg_pg.mL | Interferon gamma concentration (pg/mL) measured by standard clinical assay (Addenbrooke's Hosptial) |
| C3a_pg.mL | Concentration of C3 activation product C3a measured by ELISA in EDTA plasma (pg/mL) |
| C3c_ng.mL | Concentration of C3 activation product C3c measured by ELISA in EDTA plasma (ng/mL) |
| TCC_mAU.mL | Concentration of terminal complement complex measured by ELISA in EDTA plasma (mAU/mL) |
| Spike.IgG | Quantified SARS-CoV-2 spike protein IgG antibody titre determined by ELISA |
| N.IgG | Quantified SARS-CoV-2 nucleocapsid protein IgG antibody titre determined by ELISA |
| Spike.IgA | Quantified SARS-CoV-2 spike protein IgA antibody titre determined by ELISA |
| N.IgA | Quantified SARS-CoV-2 nucleocapsid protein IgA antibody titre determined by ELISA |
| Spike.IgM | Quantified SARS-CoV-2 spike protein IgM antibody titre determined by ELISA |
| N.IgM | Quantified SARS-CoV-2 nucleocapsid protein IgM antibody titre determined by ELISA |
| AUC.Spike.IgG | Area under titration curve, spike IgG ELISA at serial serum dilutions |
| AUC.Spike.IgA | Area under titration curve, spike IgA ELISA at serial serum dilutions |
| AUC.Spike.IgM | Area under titration curve, spike IgM ELISA at serial serum dilutions |
| AUC.N.IgG | Area under titration curve, nucleocapsid IgG ELISA at serial serum dilutions |
| AUC.N.IgA | Area under titration curve, nucleocapsid IgA ELISA at serial serum dilutions |
| AUC.N.IgM | Area under titration curve, nucleocapsid IgM ELISA at serial serum dilutions |
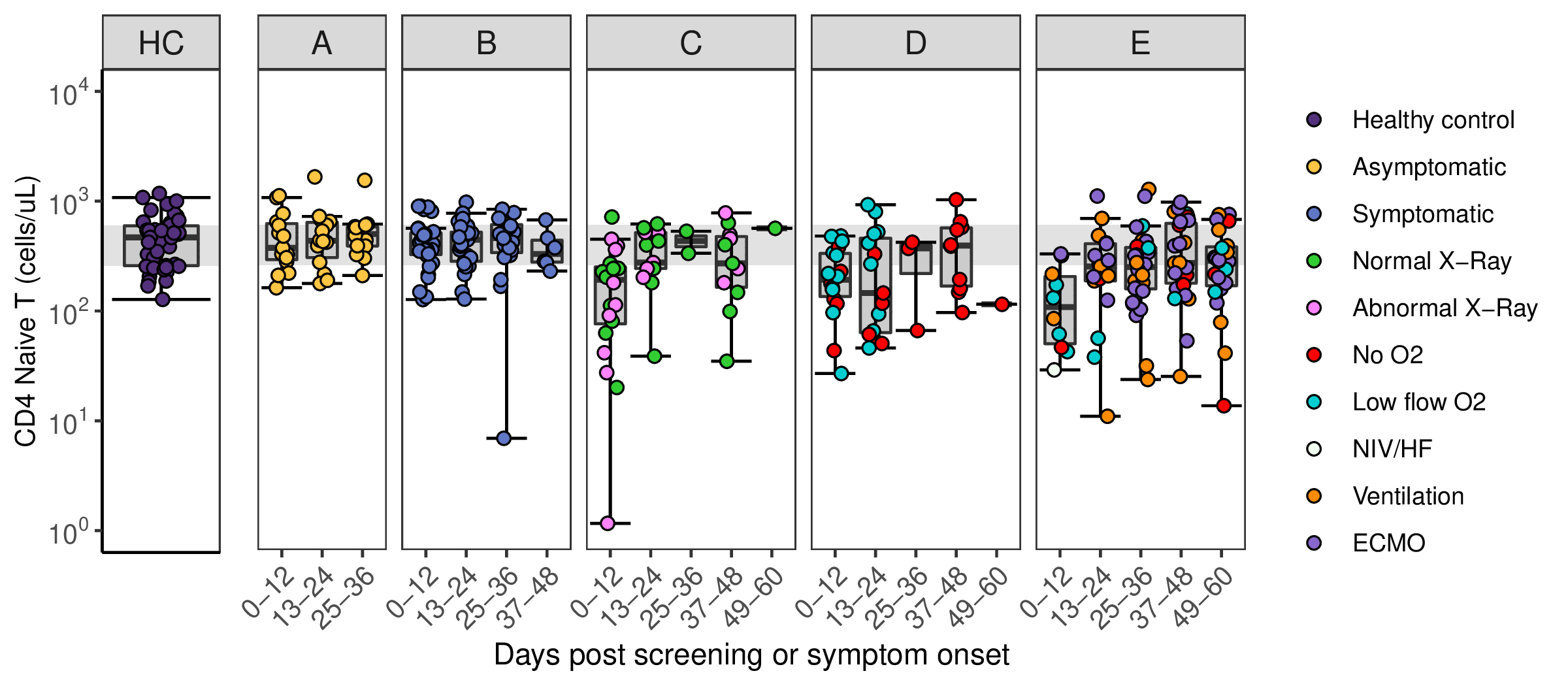
| Sample_ID | Sample ID, incorporating approximate day of follow-up from study design (see FILE 1 and 2 for metadata) |
|---|---|
| [Cell populations] | Absolute count (cells/uL) of cell populations derived from peripheral blood mononuclear cells. Measured by flow cytometry and enumerated using BD TruCount (see Methods) |
| Sample_ID | Sample ID, incorporating approximate day of follow-up from study design (see FILE 1 and 2 for metadata) |
|---|---|
| [Cell populations] | Cell proportions as percentage of parent population in whole blood Measured by mass cytometry (see Methods) |
Normalised gene expression counts derived from RNA sequencing of whole blood PAXGene tubes, Sample ID as columns, gene IDs as rows.